

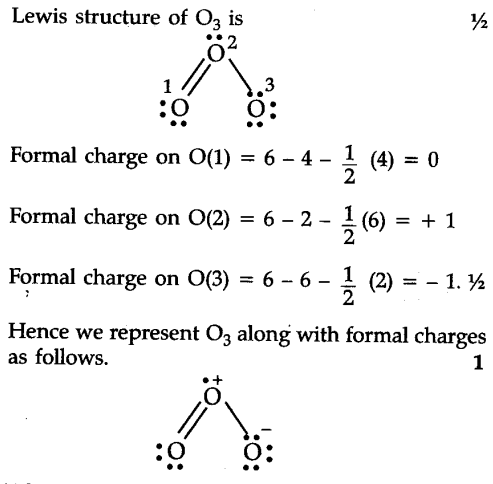
Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign.If the Lewis structure must have nonzero formal charges, the arrangement with the smallest nonzero formal charges is preferable.A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero.A few guidelines involving formal charge can be helpful in deciding which of the possible structures is most likely for a particular molecule or ion: In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure-different multiple bond and lone-pair electron placements or different arrangements of atoms, for instance. The arrangement of atoms in a molecule or ion is called its molecular structure. Using Formal Charge to Predict Molecular Structure This gives the formal charge:Br: 7 – 7 = 0Cl: 7 – 7 = 0All atoms in BrCl 3 have a formal charge of zero, and the sum of the formal charges totals zero, as it must in a neutral molecule.ĭetermine the formal charge for each atom in NCl 3. Subtract this number from the number of valence electrons for the neutral atom.Now each Cl atom has seven electrons and the Br atom has seven electrons. Assign one of the electrons in each Br–Cl bond to the Br atom and one to the Cl atom in that bond:.Thus, we calculate formal charge as follows:Īssign formal charges to each atom in the interhalogen molecule BrCl 3. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule.

#Calculating formal charge lewis structure how to
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. Explain the concept of resonance and draw Lewis structures representing resonance forms for a given molecule.Use formal charges to identify the most reasonable Lewis structure for a given molecule.Compute formal charges for atoms in any Lewis structure.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
